A traditional clinical database platform (CDB) is built upon a relational database system and generally is equipped with powerful functions for query management and tracking capability. A modern CDM in the age of electronic data capture (EDC) additionally provides user-friendly interface for site users, i.e. site coordinators, investigators and site monitors etc. All these features support data management activities (DM) on an ongoing basis and timely access to real-time clinical data for analyses and pre-planned business decisions. None of these features are readily available in a SAS environment.
However SAS programming plays a unique and important role in supporting DM [1][2]. SAS is a language built for “specialty” programmers. A person without coding background would have difficulty using it; a programmer without domain knowledge (such as clinical trial knowledge for clinical SAS programmers) would also find it difficult to use, e.g. writing a piece of code to cross check data consistency between adverse events (AE) and concomitant medications (CM) panels/data sets without first understanding the clinical relevance of the two data sets.
Cross panel checks – too much work in CDB
It is generally a time consuming process to develop complex cross-panel checks in CDB, e.g. the checks between lab data (LB) and AE. Most CDBs don’t seem to provide an easy-to-use tool that may link panels at the Visit and/or Event levels. For CDBs that allow in-between dynamic form links the difficulty is mitigated by selecting certain key information that is dynamically populated at data entry, e.g. identifying the associated AE number from a pull-down list (retrievable through pre-built link with the AE page(s)) while entering CM page. However, for CDBs that don’t have such advanced features, custom codes/solutions may have to be developed for a considerable amount of time by an experienced CDB designer/developer. In either situation, the solutions will add cost and are limited since only working for a particular type of cross panel checks. For example, the dynamic form link approach won’t work for LB and AE checks for studies that LB data is uploaded instead of being entered at the Site(s).
On the other hand, SAS is extremely useful and handy to develop edit checks on complex logic checks. What seems to be difficult to link panels at the given Visit and/or Event levels is implemented easily by simple data steps that merge the corresponding data sets by the order variables that include Visit Number and Event or Record Sequential Number etc. By doing this, an advantage is added by avoiding the heavy workload on change control process had it been totally developed in CDB.
Combining CDB and SAS provides an excellent and cost-effective solution
Therefore, combing CDB and SAS provides an excellent and cost-effective solution for the current edit check requirements that involve complex cross-panel logics. Obviously in this two-party environment, CDB is the hosting environment that hosts all DM activities including most edit checks. SAS plays a supplemental role that specifically addresses complex edit checks. It is important to update information consistently between CDB and SAS [2]. It should:
- Avoid duplicates: SAS should only present incremental information to CDB to follow up with query actions, i.e., queries that were fired previously should not be re-fired. This includes queries that have been fired but closed manually
- Avoid confusion: queries that no longer fire in SAS due to CDB data updates should be closed in CDB promptly
The ideal process
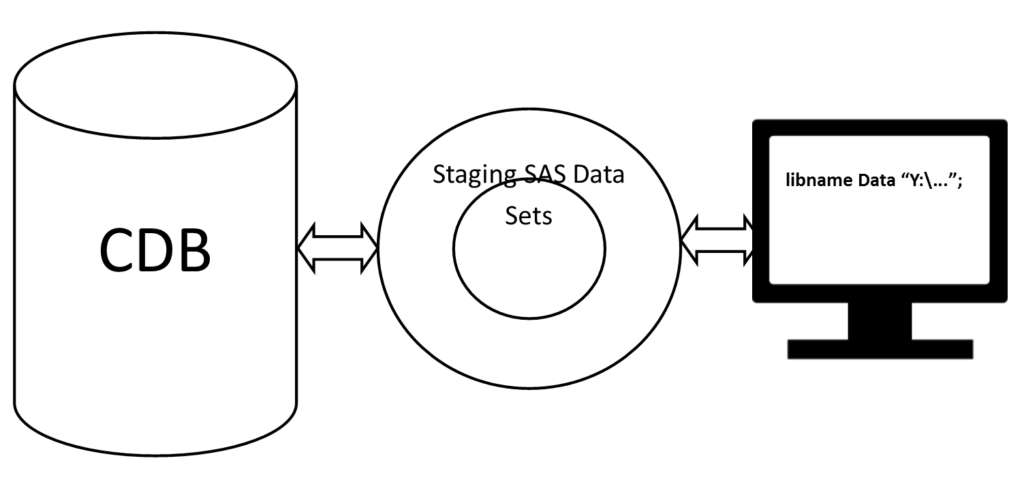
An ideal process is to establish a staging environment that stores and reconciles information from both CDB and SAS on an ongoing basis. It can be a file in Excel format or SAS format or other format. The methodology presented in [3] can be generalized for this staging environment:
Conclusion
Using the hybrid technique to satisfy the requirements of complex edit checks in CDB is a viable and cost-saving approach. SAS proves to be an effective programming language to supplement the programming functions of CDB developer/designer thanks to the powerful data manipulation capability.
References
[1] Gupta, S. Standards for Clinical Data Quality and Compliance Checks, available at www.sassavvy.com
[2] N.E.A.T._Abstract, available at sasCommunity.org
[3] Shu, H. et al, Smart Programming and SAE Reconciliation, PharmaSUG2010, paper DM06